What Is a Zone of Inhibition Test?
A Zone of Inhibition (ZOI) test is a quick way to determine the antibacterial efficacy of a treated product or surface. The method, originally known as the Kirby-Bauer test, evolved from antibiotic research in the pharmaceutical field, and was later adopted for testing the antibacterial properties of polymers and textiles.
How Is a Zone of Inhibition Test Performed?
In a typical Zone of Inhibition test, a petri dish containing a nutrient agar is streaked with the required bacteria culture. A sample of a product treated with an antimicrobial agent is cut into a piece (typically an inch by an inch) and placed onto the nutrient agar. The petri dish is then incubated for 18-24 hours at 36°C, which represents optimal conditions for bacterial growth.
After the incubation period, the bacteria on the nutrient agar should be well established and visible as a dense yellow lawn. On test pieces where the antimicrobial can migrate into the nutrient media around the sample, a clearance zone might be visible.
The size of this zone is indicative of the strength of the specific antimicrobial agent contained within the treated sample.
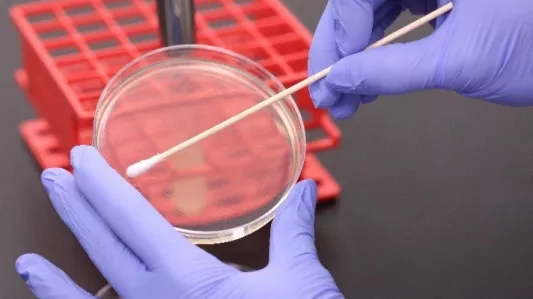
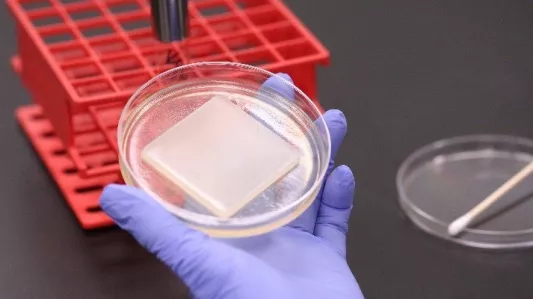
Pictured above (left): A petri dish containing a nutrient agar bed is streaked with the test bacteria. Pictured above (right): A test piece is carefully placed onto the nutrient agar containing the bacteria. The plate is then incubated for 18-24 hours at above 90% relative humidity.
What Are the Limitations of the Zone of Inhibition Test?
While the Zone of Inhibition test shows visibly compelling results, it has several limitations relating to the size of the zone.
- A large zone of inhibition does not necessarily indicate a well-protected product: a large zone of inhibition might be a sign of a leaching antimicrobial agent, whereby the active ingredient leaves a treated product and disperses into the surrounding environment. This could partly be due to gross incompatibility of the antimicrobial agent and the product itself, leading to a rapid decrease in antimicrobial performance. In addition, leaching antimicrobials may potentially expose both the user and the environment to unnecessary amounts of a biocide.
- A lack of visual zone does not mean the antimicrobial agent is ineffective: the zone of inhibition test requires the antimicrobial agent to migrate into the nutrient agar. If the antimicrobial is not compatible with the nutrient agar, it will not migrate to create a visual zone of inhibition. While it might do a very effective job in protecting the product itself, a lack of a visual zone under the Zone of Inhibition test standards might falsely lead the inexperienced evaluator to think that the antimicrobial is ineffective. Examples of effective antimicrobials that do not have a proclivity for the nutrient agar used in a Zone of Inhibition test include silver-based antimicrobials and zinc organometallic antimicrobials.
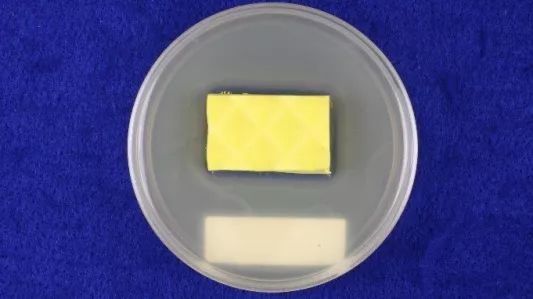
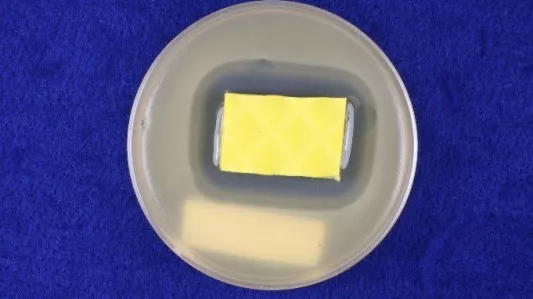
Pictured above (left & right): A test piece after incubation. The small but well-established clear zone around the sample in the left image is a sufficient indication of antibacterial efficacy. The large diffused zone around the sample in the right image might indicate more antibacterial strength, but it can also signify that the antimicrobial is not as tightly bound to the sample and has a tendency to migrate out of it.
For hazardous chemistries such as isothiazalinones, it should be considered if this migratory tendency is desired.
How Does Microban Use the Zone of Inhibition Test?
Microban uses the Zone of Inhibition test to quickly assess the performance of specific antimicrobial agents. A very large zone of inhibition is not always interpreted as superior antimicrobial product protection, but rather can be an indicator of its instability and tendency to migrate.
Typically, a small zone of clean and strong clearance around the product is desirable as an indication of antimicrobial effectiveness, longevity and stability.
While it is still commonly utilized by labs worldwide, the accuracy of the Zone of Inhibition test has largely been questioned. Today, the test method is often supplanted by more accurate quantitative methods such as ISO 22196 or JIS Z2801.
Microban Testing Services: How Can We Help You?
Microban offers a host of testing and technical support services. Our microbiology labs conduct a plethora of qualitative and quantitative tests according to recognized industry and international standards such as AATCC, ASTM, EPA, ISO, JIS, and FZ/T.
In our bacterial test lab, antimicrobial samples are evaluated for performance against Gram positive and Gram negative bacteria to determine scope of performance. Our testing portfolio includes conventional tests such as Zone of Inhibition, environmental monitoring (e.g. aerobic plate count), and Minimum Inhibitory Concentration (MIC) studies, as well as neutralization studies (ASTM E1054).
Microban's microbiology testing laboratories are certified according to ISO 9001:2008 and ISO 17025 for a wide range of antimicrobial tests. In our state of the art BSL-2 test labs, a variety of bacterial and mycological tests are routinely performed by our experienced microbiologists.
These labs have the capacity to perform over 30,000 bacterial and fungal tests annually using industry standard test methods designed for a wide range of product substrates and antimicrobial technologies.
For more information on our test capabilities and services, contact a member of the team today.
NOTE: This article may also help to explain zone size differences in a AATCC Test Method 90 test, as it is similar to a Kirby-Bauer test with some modifications.