If you ask anyone in the food industry about the challenges they face during manufacturing and processing, they will tell you that it is a hygiene-critical environment where it is vital to minimize the survival and growth of contaminating microbes. Microbial contaminants can enter the food processing chain from many sources – air, water, raw materials, equipment, pests, and people – and at various points in the process, leaving the work environment, surfaces and foods exposed to bacteria and fungi. Once microbes enter the food processing chain, they can rapidly multiply and persist on surfaces if preventive action is not taken, magnifying the effects of any hygiene lapse. This represents a major challenge for food processors, as microbial contamination can cause critical issues with product safety and quality, resulting in damaging recalls, and deterioration of the physical infrastructure of the food production facility.
A challenging environment
Maintaining microbiologically clean surfaces typically involves regular cleaning and sanitization, workflow optimization, and environmental controls. Insufficient hygiene measures can not only affect compliance with food safety standards, but may also have significant financial implications, leading to food spoilage and the destruction of potentially tainted products. In addition, microbes can cause food processing surfaces to become prematurely degraded, developing cracks, stains and odors, with considerable time and resources having to be focused on remedial actions.
To ensure products and consumers are protected, the food industry is highly regulated, with food manufacturers required to demonstrate that effective interventions are in place to limit the entry of microbes into the facility and microbial persistence, as well as the potential for cross-contamination. To this end, the U.S. Food and Drug Administration (FDA) performs 3,500 human food inspections annually, alongside another 4,000 state-contracted inspections. A recent report by the Association of Food and Drug Officials1 highlighted the outcomes of 1,262,668 state inspections and 355,772 laboratory analyses conducted in 2019, which resulted in 37,731 compliance actions. Most importantly, these inspections resulted in 1,604,817 lbs of adulterated food – worth an estimated $31,383,272 – being embargoed or quarantined, underlining the importance of good hygiene practices to the sector.
‘Always on’ antimicrobial technology
Food producers face the daily challenge of maintaining microbiologically clean surfaces, dealing with the continuous introduction of microbial contaminants, limited access to some surfaces, and diminishing chemical activity of cleaning materials over time post application. This is where built-in antimicrobial technologies come to the fore. Registered and regulated antimicrobials can be incorporated into both food and non-food contact surfaces, providing support for the conventional hygienic practices of cleaning, sanitizing and disinfection. Because these antimicrobial technologies are built in, they don’t wear off or wash away, working 24/7 for the lifetime of the product.
A silver lining
A recent poll of webinar participants2 revealed that 40 % considered tiling on countertops, floors, and walls to be the most important application for built-in antimicrobial technology, followed by air systems – filters, ductwork, insulation – at 30 %. Silver has long been used as an antimicrobial, for example, to dress wounds and treat burns. It has a broad range of antimicrobial activities – bacteria, yeast and molds are generally susceptible – is effective at low concentrations (<1 ppm) and has minimal effects on human cells. Today, silver is used throughout food processing industry for applications including air filters, taps, doors frames and handles, countertops, conveyors, wall and floor tiles, and sanitary ware.
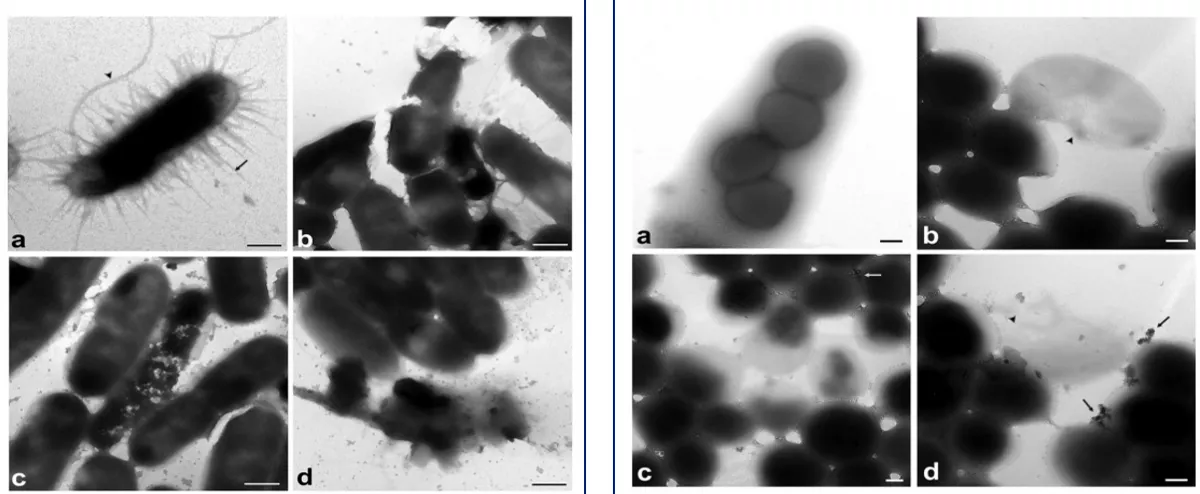
External morphologies of E. coli (Left) and S. aureus (Right) observed by TEM
(a)Untreated bacteria; (b), (c), (d) Bacteria treated with silver ion solution, 0.2 ppm
Jung et al. 2008
Built-in antimicrobial technologies, such as Microban® SilverShield® technology, take advantage of silver’s antimicrobial properties to provide durable and complementary surface protection against bacteria, mold, and mildew growth. Microban antimicrobial technology works at a cellular level, continually disrupting the growth and reproduction of microorganisms. It operates a multi-modal attack on the microbe, disrupting protein activity and DNA replication, and destroying the bacterial cell wall and membrane to cause cell dehydration. This ‘always on’ technology can be built in at the time of manufacture, becoming a permanent feature that remains active for the product’s expected lifetime, working alongside regular cleaning protocols to help manufacturing facilities maintain hygienically cleaner food processing environments.
Species | Agent | D-Value |
| Escherichia coli | Ag+ (10-5) | 4.1 4.9 |
| Pseudomonas aeruginosa | Ag+ (10-5) AgNO3 (10-5) | 38.7 62.5 |
| Caenorhabditis albicans | Ag+ (10-6) AgNO3 (10-6) | 62.5, 30.0 |
| Aspergillus niger | Ag+ (10-6) AgNO3 (10-6) | 47.6 |
Table: Microbiocidal Activity of Ag+ and AgNO3
Meeting safety, efficacy, and regulatory requirements
Microban SilverShield technology is rigorously tested using standard industry methods – ASTM E2180, E3031 and G21, ISO 22196 and JIS Z2801 – to demonstrate the efficacy required for registration, as well as undergoing chemical analysis for quality assurance. But this is just the start. Food manufacturers around the world must comply with a plethora of safety regulations that apply to both food ingredients and food contact materials, as well as antimicrobial material preservatives and antimicrobials intended for food contact.
Treated articles are governed by regulations such as US EPA PR Notice 2000-1, Article 58 of EU BPR 528/2012, and the Canada PMRA Information Note, as well as international advertising laws. Microban SilverShield technology includes direct and indirect food contact permissions on EPA labels for drinking water, food service and food preparation applications, and has Article 95 Product Type 4 (food contact) listing and EFSA opinion permitting use in food contact applications. This is backed by a history of safe use in medical devices, and food and beverage applications.
Protection from bacteria and fungi may be the main aim of implementing built-in antimicrobial technology but, in today’s environmentally conscious world, there is an ever-increasing focus on sustainability. Built-in antimicrobial technologies can also help to reduce waste by minimizing food contact with non-hygienic surfaces, as food that may have been contaminated must be disposed of. Water consumption is also decreased, since the need for continuous cleaning to maintain the hygienic environment is lessened, and production efficiency is improved, as less manpower is diverted to cleaning activities. So, however you look at it, from antimicrobial protection to greener workflows, the implementation of built-in technologies in the food industry is a win-win situation.
1 Association of Food and Drug Officials 2020-2021 State Food Program Resource Survey.
https://www.afdo.org/wp-content/uploads/2021/09/Resource-Survey-Summary-Report-Final.pdf
2 Xtalks webinar: Utilizing built-in antimicrobial technology to support surface hygiene and cleanliness in the food processing environment. Glenner M. Richards, Ph.D., Director of Microbiology and Analytical Chemistry, and Tara Conley, Director of Regulatory Affairs. October 12, 2021.


